 Research Article
Research Article
The Acute Effect of N-Acetylcysteine Supplementation on Repeat Sprint Performance
Miltenberger M1*, Zipp G2, Parasher R3 and Davis S1
1East Stroudsburg University of Pennsylvania, USA
2Seton Hall University, USA
3Director, Amar Jyoti Institute of Physiotherapy, USA
Matthew Miltenberger, East Stroudsburg University of Pennsylvania, USA
Received Date:October 16, 2024; Published Date:April 21, 2025
Repeat sprints are the main mode of locomotion in most fields and court-based sports. During these movements there is an increased load placed on the aerobic energy system to aid in ATP production and recovery. This up regulation increases fatiguing factors such as Reactive Oxygen Species (ROS), N-Acetylcysteine (NAC) has been shown previously to scavenge ROS during aerobic exercise but to date the effects of acute supplementation on repeat sprint activities in unclear.
Objective: This study was designed to investigate the effect of acute NAC supplementation on repeat sprint performance.
Methods: Eighteen recreationally active male college students volunteered to participate in this study. Each subfecundities’ the repeat sprint
protocol, 12x30 meters with 35 seconds of passive recovery in 3 different conditions; the control trial which consisted of no pretreatment before
sprints, the placebo which required the subjects to ingest 150ml of water mixed with crystal light lemonade drink mix® approximately 90 minutes
before sprinting and the experimental trial, in which subjects ingested the 150ml crystal light solution mixed with 70mg/kgbtw of NAC powder 90
minutes prior to the start of testing.
Results: Statistical analysis (ANOVA) revealed no statistically significant difference between the control, placebo, and experimental condition for mean sprint time, peak sprint time, and fatigue index, p<0.05 for all measures. Of particular interest, mean sprint time by condition showed no descriptive difference with mean values of 4.72 ± .18, 4.72 ± .21, and 4.73 ± .19 for control, placebo, and experimental trials respectively.
Conclusion: The findings of this study suggest that NAC supplementation of 70mg/kg btw has no effect on repeat sprint performance, specifically mean sprint time, peak sprint time, and fatigue index. Further research is warranted to explore prolonged supplementation, the type of recovery between sprints, and time spent in recovery.
Keywords:Fatigue; Reactive Oxygen Species; Peak Power; Mean Power
Introduction
Repeat sprints are the main mode of locomotion in the field and court-based sports such as rugby, field hockey, basketball, and soccer. They can be defined as repeated bouts of near maximal activity interspersed with short recovery periods [1]. Repeat sprints are physiologically complex due to the requirement of high power output paired with rest times that do not sufficiently allow the anaerobic phosphocreatine (PCr) energy system to recover fully before the subsequent sprint occurs. Previous research suggests that due to incomplete recovery of the PCr system other metabolic resources are upregulated to aid in ATP provision. Bogdanis et al., 1996 [2] found after repeated 30 second Wingate sprints with 4 minutes of recovery that the anaerobic system did not recover completely, and the aerobic system contributed a significant amount of energy. Sander et al., 2017 [3] found a significant negative correlation between RSA and aerobic capacity suggesting aerobic fitness is associated with faster sprint times. The utilization of multiple energy systems allows the main tense of performance over time, however, there tends to be a progressive decline in performance as sprints work continues indicating multiple factors of fatigue come into play [1].
Although there are many factors that play into fatigue during repeat sprint activities, there is body research that suggests free radicals, specifically reactive oxygen species (ROS) significantly impact working muscle’s ability to contract [4]. To counteract this ROS effect, antioxidants play an important role in neutralizing free radicals [5,6]. There are a variety of antioxidants available each having a separate role in the reduction of free radicals. Data suggests that for protection against oxidative damage and related diseases a wide variety of antioxidants should be ingested through a diverse selection of foods [7]. Interestingly, Machefer, et al., 2007 [8], studied the antioxidant status of 19 ultra-endurance athletes before competition. The researchers collected a 7-day diet log leading up to the race, upon evaluation they determined all subjects had insufficient energy intake. They also noted that 18 subjects had low antioxidant vitamin E intake, and 6 subjects had low antioxidant vitamin C and beta-carotene intake. They concluded that insufficient caloric energy intake was responsible for insufficient antioxidant intake, thus providing insight into the athletic diet. Although dietary food ingestion provides the best availability for antioxidant absorption it may not be plausible to consume enough calories to meet these needs. This provides some efficacy for the supplementation of antioxidants in the athletic population. Without sufficient intake of not only macronutrients but also antioxidants performance may not be maximized.
There are an abundant number of antioxidants commercially available, each having their own specific reported benefits. Of specific interest is N-Acteylcysteine (NAC), a thiol containing compound that can target ROS. Thiol is a class of organic sulfur derivatives that have many functions in the body including a central role in antioxidant defense [9]. NAC is considered one of the most beneficial non-specific thiols donating antioxidants. N- Acetylcysteine’s chemical formula is C5H9NO3S. It is rapidly absorbed after oral dose and broken down into a variety of metabolites. Peak concentrations of NAC appear in plasma less than 1 hour after ingestion with the plasma half-life being approximately 2.15 hours. In most cases no NAC can be detected in the system 10- 12 hours post ingestion [10,11]. The major metabolites associated with NAC breakdown are cysteine and inorganic sulfite. Cysteine is an amino acid that along with glycine and glutamic acid form the antioxidant glutathione. Cysteine is a non-essential amino acid and is the rate limiting step for glutathione production [13]. Increases in cysteine availability can upregulate glutathione production during times of increased oxidative stress since only low amounts of glutathione are stored and it is synthesized when needed from amino acid supplies [11]. This concept is demonstrated by the therapeutic administration of NAC after Acetaminophen overdose. In high doses acetaminophen depletes glutathione levels and inhibits cytosolic glutathione transferase activity. If NAC is administered within 1 hour of overdose both of these effects can be prevented. This suggests that NAC has a rapid supportive effect for glutathione production after acetaminophen overdose [13,11]. Medved et. al., 2004 [6] researched the effect of NAC on the attenuation of fatigue in aerobically endurance trained athletes. Eight male subjects completed a submaximal aerobically based cycle ergometer test consisting of pedaling to exhaustion before and after NAC supplementation. During the experimental trial NAC was intravenously applied before and during exercise, blood and muscle biopsies were taken before during and after the exercise protocol. Results showed that NAC was effective in elevating blood glutathione levels, increasing time to exhaustion as well as total work done, therefore increasing performance. The researchers also reported no adverse reactions to the supplement. Lee, West, Phillips, and Britz-McKibbin, 2010 found similar results using high dose oral NAC supplementation during a prolonged aerobic cycle ergometer test to exhaustion. The subject performed a maximal exercise test consisting of pedaling for 45 minutes at 70% of VO2 peak then pedaling at 90% of VO2 peak until exhaustion. The experimental protocol consisted of 5 days of oral NAC supplementation at 75mg/ Kg of body weight, diluted in 250 ml of water with 1 low calorie packet of crystal light for flavor. During supplementation the subject reported no adverse side effects. After 5 days the subject repeated the exercise protocol. Blood samples were collected before, during and after exercise along with exercise data. The results show a 14% decrease in red blood cell oxidation, a shorter recovery time after exercise, and greater time to fatigue due to lower oxidative stress markers. This study suggests that NAC can be effectively taken orally with the same beneficial effects to exercise as intravenous supplementation. Further research of pro-antioxidant status was performed by Zembron-Lancy, Slowinska-Lisowska, Szygula, Witkowski, & Szyszka, 2010. The researchers found that after eight days of NAC supplementation at approximately 1200mg per day, glutathione levels were increased by 31% as well as hemoglobin levels increased 9%, erythropoietin levels increased 26%, and levels of oxidative damage markers decreased by more than 30%. Although this demonstrates a proantioxidant status the researchers concluded that supplementation did not increase incremental exercise to exhaustion performance. In comparison to other research, the supplementation levels of 1200mg were comparatively much lower to those found in the research carried out by Lee, West, Phillips, & Britz-McKibbin, 2010, who used supplementation dosage as a function of body mass (75mg/ kg of body weight). The body mass formula used higher dosages for their subjects and found positive results for attenuating fatigue and increasing aerobic exercise performance.
Since repeat sprint activity is the main mode of locomotion in most field and court-based sports, and during these times of high intensity movements with short recovery periods there is an upregulation of aerobic metabolism it would come to reason that there would be an increase in oxidative stress via ROS. The use of NAC as a nutritional supplement to scavenge ROS may be beneficial to these athletes. Therefore, the purpose of this study was to evaluate the effectiveness of the antioxidant N-Acetylcysteine (NAC) in the attenuation of fatigue during a repeat sprint protocol. This study specifically investigated the implications of NAC with respect to fatigue using the percent decrement formula as well as changes in peak sprint time and mean sprint time.
Methods
Eighteen recreationally active male college students (Age 20.3 years ±1.4, Height 177.9 cm ± 7.33, Mass 71.5 kg ± 7.3) volunteered to participate in this study. All subjects had a previous history of playing or competing in repeat sprint-based sports, were currently free from injury that would inhibit physical performance of the repeat sprint protocol and were not currently (previous 3 months) taking any nutritional supplements including multivitamins and antioxidants. Prior to participation all subjects completed a health history form, PAR-Q, and informed consent. The experimental protocol was approved by the East Stroudsburg University Institutional Review Board for the protection of Human Subjects.
Subjects voluntarily participated in 3 counterbalanced trials, control, placebo, and experimental with a 7-day washout between each. Each subject was blinded for the placebo and experimental conditions to avoid bias. On the day of testing, subjects reported to the University Human Performance Laboratory approximately 120 minutes prior to the start of the sprint protocol, at this time each subject was fitted with a Polar ® FT7 heart rate monitor and asked to quietly rest. Approximately 20 minutes prior to beginning testing baseline measurements were collected including heart rate, resting lactate (Lactate Pro ®), as well as RPE. The subjects were then asked to perform a standardized warm-up consisting of 3 minutes of jogging, and 12 minutes of dynamic stretches for all the major muscle groups of the lower extremity. Once completed the subjects were given a 2-minute rest period followed by the repeat sprint protocol which consisted of 12 x 30-meter sprints interspersed with 35 seconds of passive recovery [14]. The sprints took place on an indoor track that was climate controlled. A Brower® wireless timing system was used to record times of each sprint, RPE and Heart rate were collected after the completion of even numbered sprints, and Lactate was collected within 30 seconds of completion of the final sprint. During the control condition the subjects did not receive any pre-treatment beverage nor ingested any food or drink for 120 minutes prior to the sprint protocol. In the placebo condition subjects ingested Crystal Light® lemon flavored drink powder dissolved in 8oz of water 90 minutes prior to the start of the repeat sprint protocol. The solution was placed in an opaque plastic cup with a lid which concealed the smell and visual appearance. The subjects were told to consume the beverage within 5 minutes of receiving it. The experimental condition included the addition of NAC (nutrbio®) to the pre-treatment solution. Subjects were given the same Crystal Light ® lemon flavored powder in 8oz of water as well as 70/mg/kg of pharmaceutical grade NAC [15].
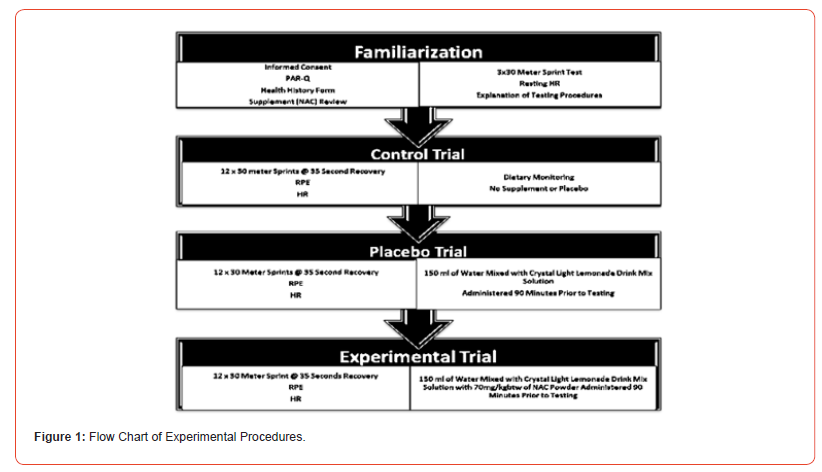
Figure 1 shows the procedural steps for data collection, the three conditions were counterbalanced with randomization of subjects into groups. Each trial was separated by a 7 day wash out period.
Statistical Analysis
All statistical analyses of the data were carried out using SPSS 23® (IBM, Armonk, NY, USA).
Results
Statistical analysis (ANOVA) revealed no statistically significant difference between the control, placebo, and experimental condition for RPE, heart rate and fatigue index. This result suggests that during each of the trials subjects exerted similar effort. Mean heart rate (bpm) was 179.80, 177.77, and 177.19 for control, experimental and placebo conditions respectively. Similar results were seen for RPE of 13.33, 12.76, and 12.67 across conditions. Finally, fatigue index (FI) calculated through the percent decrement formula was exceptionally low across all conditions for repeat sprint movements. Fatigue index values (%) were 5.79, 5.60, and 5.66.
Table 1:Descriptive Statistics for Sprint Times.
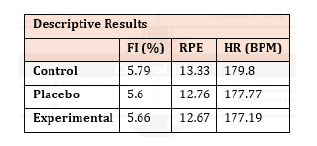
Figure 2 represents the descriptive results for Fatigue Index (FI), rating of perceived exertion (RPE), and heart rate (HR). Further analysis showed no statistical difference between the three conditions.
Sprint data, mean sprint time and peak sprint time, showed no statistically significant difference (p>0.05) across all conditions. Although it was hypothesized that peak or fastest sprint time would be influenced very little by NAC due to peak sprint time occurring early on in the protocol (sprint 2) before significant onset of oxidative stress. However, of particular interest was mean sprint time or the average time to complete all 12 sprints in the trial. It was hypothesized that NAC would lower mean sprint time through the attenuation of fatigue allowing subsequent sprints to be faster, therefore leading to lower mean sprint times. Statistical analysis revealed no difference between conditions with mean values of 4.72 ± .18, 4.72 ± .21, and 4.73 ± .19 for control, placebo, and experimental trials respectively.
Figure 3 depicts the results of peak or fastest sprint time by condition, further statistical analysis revealed no significant difference across conditions.
Figure 4 is the mean or average sprint time across all 12 sprints by condition. There was no significant difference in mean sprint time by condition.
Discussion
Understanding the complexity of repeat sprint work is of the upmost importance to maximize performance, specifically attenuating the onset of fatigue would be exceptionally valuable for the maintenance of sprint parameters over time. The purpose of this study was to investigate the effect of NAC, an antioxidant which promotes the scavenging of ROS, in attenuating fatigue during repeat sprints. Heart rate data suggested that across all conditions there was no significant difference in subject adherence to the protocol, during all three conditions the average heart rate across trials was between 89-90% of age predicted maximum heart rate. Similarly, RPE showed no significant differences across conditions as well averaging between 12.67-13.33/ 20. The importance of this data is two-fold, first it demonstrates consistency of effort by subjects, significant differences in heartrate or RPE between conditions would make it difficult to contribute changes in performance in relation to supplementation or placebo if different level of effort was given. Interestingly, although the conditions were similar compared to previous repeat sprint research heartrate and associated RPE’s were much lower than anticipated, this is confirmed by the fatigue index scores between 5.60 and 5.79%. The fatigue score would suggest that’s sprint performance across all 12 trials diminished by only a small percentage, compared to similar studies where fatigue ranged between 7.1 to 27% after 6 weeks of training with varied recovery periods [16]. The low fatigue rate in this study suggests that the repeat sprint protocol may have been used to large of work to rest ratio, allowing for greater recovery between sprints. The lack of fatigue perceived or measured may neutralize any benefit of NAC supplementation.
Similar to heartrate and RPE data indices of repeat sprint ability suggests that there was no difference between peak (fastest) or mean (average) sprint time between control, placebo, or experimental conditions. As stated previously, there does not seem to be a mechanism for NAC to effect peak sprint time due to this occurring typically during the first few sprints as a function of force development instead of relating to the attenuation of fatigue as in mean sprint time. Focusing on mean sprint time, it was hypothesized that the NAC supplementation group would show lower mean sprint times, in other words the subjects during the NAC supplementation would be able to maintain faster sprint times across all 12 sprints, lowering their average. Medved et al., 2004, used intravenous NAC supplementation and improved time to fatigue, Lee et al., 2010 [17] also saw attenuation of fatigue after 5 days of supplementation. The results of this study do not agree with those results; however, the current study used an acute oral dose and may not have created a pro-antioxidant status as measured by the previous studies. An oral loading phase of NAC or the introduction of NAC through intravenous means may be more advantageous in the attenuation of fatigue during repeat sprints.
Conclusion
Although a promising theoretical frame exists for the support of NAC as a nutritional supplement to aid in the attenuation of fatigue in repeat sprint athletes, current data does not support this concept. Future recommendations include using a repeat sprint protocol with a greater volume of sprints, a shorter recovery time that mimics those found in field and court sports, and finally using an active recovery between sprints instead of a passive static recovery. The use of a protocol that more resembles a sport like pattern may in fact show some efficacy for an acute oral dose of NAC to attenuate fatigue.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Glaister M (2005) Multiple Sprint Work. Sports Medicine 35(9): 757-777.
- Bogdanis G, Nevill M, Boobis L, Lakomy H (1996) Contribution of Phosphocreatine and Aerobic Metabolism to Energy Supply During Repeat Sprint Exercise. Journal of Applied Physiology 80(3): 876-884.
- Sanders GJ, Turner Z, Boos B, Peacock CA, Peveler W, et al. (2017) Aerobic Capacity is Related to Repeated Sprint Ability with Sprint Distances Less Than 40 Meters. Int J Exerc Sci 10(2): 197-204.
- Allen D, Lamb G, Westerblad H (2007) Impaired Calcium Release During Fatigue. Journal of Applied Physiology 104(1): 296-305.
- Zembron-Lancy A, Slowinska-Lisowska M, Szygula Z, Witkowski Z, Szyszka K (2010) Modulatory Effect of N-acetylcysteine on pro-antioxidant status and haematological response in healthy men. Journal of Physiology and Biochemistry 66(1): 15-21.
- Medved I, Brown M, Bjorksten A, Murphy K, Peterson A, et al. (2004) N-Acetylcysteine enhances muscle cysteine and glutathione availabiity and attenuates fatigue during prolonged exercise in endurance trained individuals. Journal of Applied Physiology 97(4): 1477 -1485.
- Jacob RA, Burri BJ (1996) Oxidative damage and defense. Am J Clin Nutr 63(6): 985S-990S.
- Machefer G, Groussard C, Zouhal H, Vincent S, Youssef H, et al. (2007) Nutritional and Plasmatic Antioxidant Vitamins status of Ultra Endurance Athletes. Journal of the American College of Nutrition 26(4): 311-316.
- Sen K, Packer L (2000) Thiol Homeostasis and Supplements in Physical Exercise. American Journal of Clinical Nutrition 72(2 Suppl): 653-695.
- De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, et al. (1989) Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung 39(3): 382-386
- Kelly G (1998) Clinical Applications of N-Acetylcysteine. Alternative Medicine Review 3(2): 114-127.
- Whillier S, Raftos JE, Chapman B, Kuchel PW (2009) Role of N-acetylcysteine and cystine in glutathione synthesis in human erythrocytes. Redox Rep 14(3): 115-124.
- Pratt S, Ioannides C (1985) Mechanism of the protective action of n-acetylcysteine and methionine against paracetamol toxicity in the hamster. Arch Toxicol 57(3): 173-177.
- Glaister M, Howatson G, Lockey R, Abraham C, Goodwin J, et al. (2007) Familiarization and Reliability of Multiple Sprint Running Performance Indices. Journal of Strength and Conditioning Research 21(3): 857-859.
- Ferreira LF, Campbell KS, Reid MB (2011) N-acetylcysteine in handgrip exercise: plasma thiols and adverse reactions. Int J Sport Nutr Exerc Metab 21(2): 146-154.
- Glaister M, Stone M, Stewart A, Hughes M, Moir G (2007) The Influence of Endurance Training on Multiple Sprint Cycling Performance. Journal of Strength and Conditioning Research 21(2): 606-612.
- Lee R, West D, Phillips S, Britz-McKibbin P (2010) Differential Metabolomics for Quantitative Assessment of Oxidative Stress with Strenuous Exercise and Nutritional Intervention: Thiol Specific Regulation of Cellular Metabolism with N-Acetyl-L-Cysteine Pretreatment. Analytical Chemistry 82(7): 2959-2968.
-
Miltenberger M*, Zipp G, Parasher R and Davis S. The Acute Effect of N-Acetylcysteine Supplementation on Repeat Sprint Performances. Aca J Spo Sci & Med. 2(4): 2025. AJSSM.MS.ID.000546.
-
N-Acetylcysteine, Sprint Performance, Acute Effect, Reactive Oxygen Species, Peak Power; Mean Power, Fatigue
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






